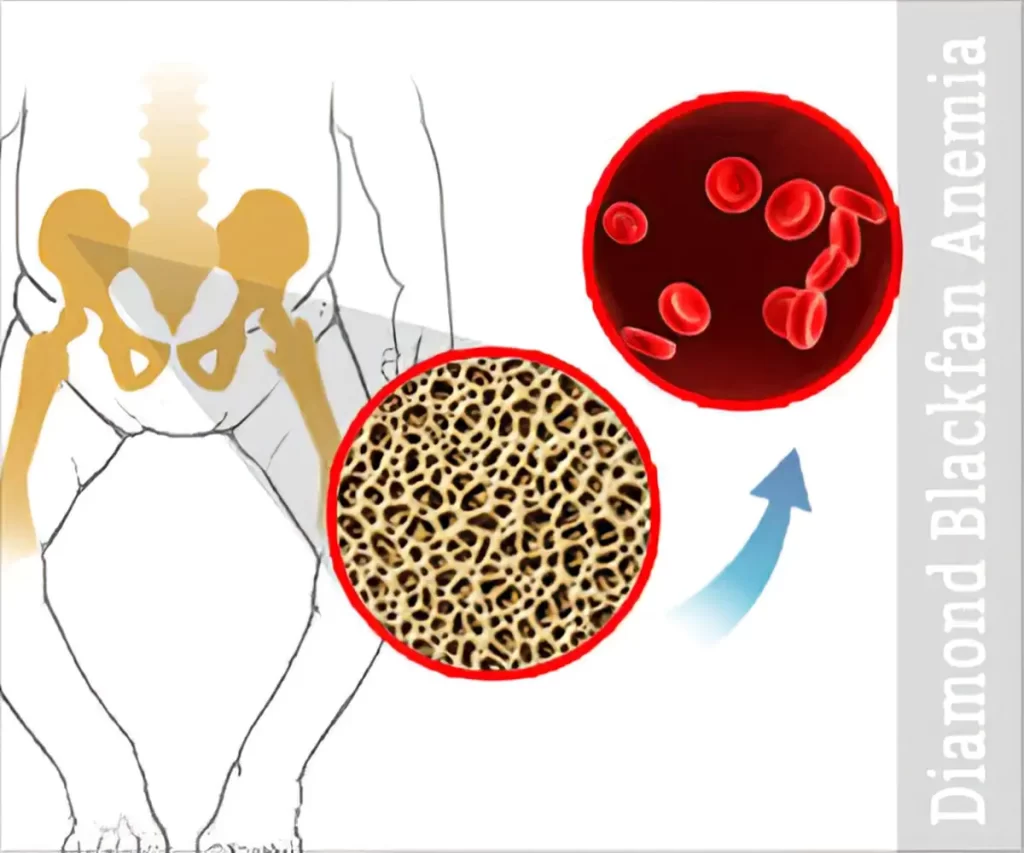
What is Diamond Blackfan Anemia?
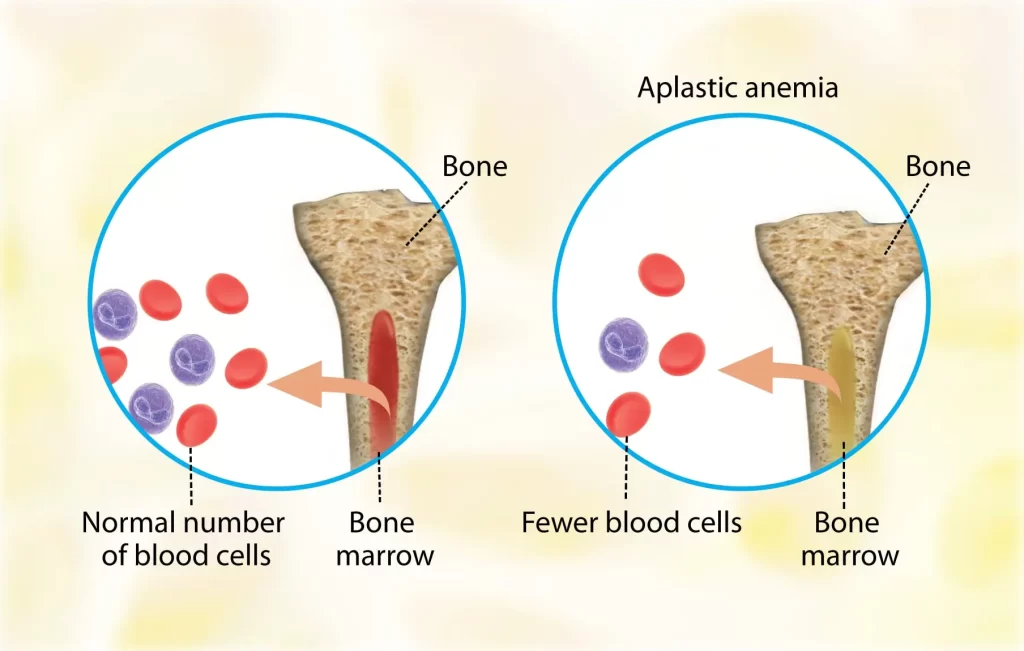
Diamond Blackfan anemia (DBA) is a form of congenital anemia that is characterized by pure red cell aplasia and is related to congenital bone abnormalities.
It is a type of macrocytic-normocytic anemia that is chronic.
DBA is a genetic disease that is inherited as an autosomal dominant inheritance in 40 to 45% of cases. The remaining cases, which account for 55 to 60% of all cases, typically present sporadically.
Causes of Diamond Blackfan Anemia
Diamond Blackfan anemia develops as a result of a genetic mutation.
The most common mutation affects a ribosomal protein on chromosome 11. However, newer studies have discovered mutations in the transcription factor GATA1.
In between 60 and 70 percent of DBA cases, ribosomal gene mutations are more prevalent and present.
It involves the gene responsible for producing both small and large ribosomal protein units, including but not limited to RPL5, RPL11, RPS 7, RPS 17, RPS 24, RPS 10, RPS 19, and RPS 26.
Mutations in the RPL3, RPL7, RPL14, RPL19, RPL26, RPL36, RPL23A, RPL35, RPS 15, RPS 8, RPS27A, RPL35, and RPL18 genes have been observed in some families.
Even though DBA has a significant genetic component, 30 to 35% of cases are genetically indeterminate.
Defective ribosomal protein biosynthesis causes apoptosis in erythroid progenitor cells via p53 activation and stabilization, resulting in erythroid failure.
This is referred to as ‘ribosomal stress.’ Up to 50% of DBA cases have a ribosomal gene heterozygous mutation with loss of function.
Epidemiology of Diamond Blackfan Anemia
Diamond Blackfan anemia is a very rare disease that affects 1 in 500000 live births. In most cases, onset occurs within the first year of life.
The incidence of DBA is similar across ethnicities and genders.
Pathophysiology of Diamond Blackfan Anemia
DBA is a chronic form of macrocytic-normochromic anemia characterized by low reticulocytes, normal platelets, and normal WBCs in the bone marrow.
The most frequently mutated ribosomal protein gene is RPS19, which codes for a small ribosomal protein unit and is present in about 25% of DBA cases.
RPS gene mutations include deletions, insertions, nonsense, splice sites, and frameshift changes.
These mutations cause a deficiency of RPS19 protein, which is known as ‘haploinsufficiency’.
RPS19 mutation reduces RPS19 and 40S ribosomal subunit production by reducing translation initiation.
The normal differentiation and production of primary hematopoietic progenitor cells are negatively impacted by the increased apoptosis that results from the gene’s decreased initiation of translation.
Overexpression of exogenous RPS19 can compensate for RPS19 deficiency, which helps explain the haploinsufficiency phenomenon and suggests that DBA may be treatable with RPS gene augmentation.
According to the ‘ribosomal stress’ mechanism, ribosomal deficiency activates and stabilizes p53, subsequently initiating apoptosis and causing bone marrow failure by terminating cell lines.
DBA can also result from mutations in non-Rp genes like GATA1. Transcriptional factors are encoded by GATA1.
It is crucial to the differentiation of erythroid cells.
Leucine is changed into valine as a result of the GATA1 mutation of the G to C transversion on the X chromosome.
This mutation affects the GATA1 splicing process and leads to GATA1 termination, which is essential for erythroid cell differentiation.
GATA1 mutations ultimately cause a global translation blockade.
Read Also: Sickle Cell Anemia | Causes, Symptoms, Diagnosis and Treatments
Complications of Diamond Blackfan Anemia
Patients with Diamond Blackfan anemia are highly susceptible to hematological complications in the first year of life.
DBA is highly susceptible to developing AML, MDS, and solid tumors.
Iron overload can cause complications such as growth failure, organ failure, and infections, iron overload occurs as a result of blood transfusion, chronic steroid use, and HSCT.
Diagnosis of Diamond Blackfan Anemia
A. Clinical Presentation
Diamond Blackfan anemia appears in 90% of patients before the age of 12 months.
Congenital bony malformations account for 50% of cases, and growth retardation accounts for 30%.
The median age of the patient at presentation and diagnosis is two months.
Children typically first exhibit pallor and lethargy.
Patients typically present with severe macrocytic anemia and normochromic anemia, as well as erythroid aplasia due to congenital bone marrow failure.
Although leukocyte and platelet counts are typically in the normal range, there have been patients with low leukocyte and high platelet counts.
Reticulocyte counts in patients can be extremely low.
DBA has also been linked to increased fetal hemoglobin levels, erythropoietin, and eADA activities.
In 50% of cases, physical abnormalities are present.
The following are the most typical congenital physical abnormalities: –
- Thumb and upper extremity malformations.
- Craniofacial anomalies.
- Short stature.
- The patient may also have a snub nose and wide-spaced eyes.
DBA anomalies are characterized by a distinct facial appearance and triphalangeal thumbs.
The PRL5 mutation is linked to cleft lip or cleft soft palate, whereas the RPL11 mutation primarily correlates with thumb abnormalities but can also be seen in cleft lip or palate cases.
Urogenital anomalies and atrial and ventricular septal defects can also present.
Related: Pernicious Anemia | Causes, Symptoms, and Treatments
B. Diagnostic Criteria for Classical DBA
- Age of onset less than 12 months.
- Macrocytic anemia without other significant cytopenias.
- Reticulocytopenia.
- Bone marrow with normal cellularity with a lack of erythroid precursor.
Major Supporting Criteria
- Gene mutation described in DBA.
- Positive family history.
Minor Supporting Criteria
- Elevated ADA activity.
- Congenital anomalies are described in classical DBA.
- Elevated HbF.
- No evidence of another inherited bone marrow failure syndrome
Treatment of Diamond Blackfan Anemia
The first-line therapy for Diamond Blackfan anemia is corticosteroids. However, patients with DBA frequently need ongoing blood transfusions and concurrent iron chelation therapy because of the long-term side effects of corticosteroids.
When steroid therapy is effective but the side effects are intolerable, the patient will need chronic blood transfusions.
Regular serum ferritin monitoring helps in determining whether iron chelation therapy is required.
After 12 to 15 units of blood transfusions, iron chelation is typically started if the serum ferritin concentrations increase to 1000 to 1500 microgram/L or if the hepatic iron concentration increases to 6 to 7 mg of the dry weight of liver tissue.
Deferasirox and desferrioxamine are the recommended treatments for iron chelation.
To reduce the dosage of steroids and, consequently, their side effects, metoclopramide can be used in conjunction with steroid therapy.
Clinical trials have also suggested that leucine could be used as a supplemental therapy to steroids.
Hematopoietic stem cell transplants (HSCT) may also be used to treat DBA.
The hematological manifestation of DBA can only be treated using this method, but it can be risky if a sibling donor who matches the patient is not available.
Recent literature suggests that gene therapy and gene editing may be used to treat DBA in the future.
Read Also: Symptoms of Dying From Anemia
Summary
Diamond Blackfan anemia (DBA) is a form of congenital anemia that is characterized by pure red cell aplasia and is related to congenital bone abnormalities.
It is a type of macrocytic-normocytic anemia that is chronic.
Diamond Blackfan anemia develops as a result of a genetic mutation.
The most common mutation affects a ribosomal protein on chromosome 11. However, newer studies have discovered mutations in the transcription factor GATA1.
Diamond Blackfan anemia is a very rare disease that affects 1 in 500000 live births. In most cases, onset occurs within the first year of life.
Patients with Diamond Blackfan anemia are highly susceptible to hematological complications in the first year of life.
Diamond Blackfan anemia appears in 90% of patients before the age of 12 months.
Congenital bony malformations account for 50% of cases, and growth retardation accounts for 30%.
The median age of the patient at presentation and diagnosis is two months.
Children typically first exhibit pallor and lethargy.
The first-line therapy for Diamond Blackfan anemia is corticosteroids. However, patients with DBA frequently need ongoing blood transfusions and concurrent iron chelation therapy because of the long-term side effects of corticosteroids.
When steroid therapy is effective but the side effects are intolerable, the patient will need chronic blood transfusions.
Hematopoietic stem cell transplants (HSCT) may also be used to treat DBA.
Recent literature suggests that gene therapy and gene editing may be used to treat DBA in the future.
[ratemypost]
References
- Gadhiya, K., & Budh, D. P. (2020). Diamond Blackfan Anemia. PubMed; StatPearls Publishing. From PubMed