Heavy menstruation is a risk factor for iron-deficiency anemia due to the amount of iron lost per...
Read MoreMicrocytic Anemia | Types, Diagnosis and Treatments
Introduction
Microcytic anemia is caused by insufficient hemoglobin (Hb) formation in erythroid precursors, resulting in a decrease in red blood cell mean corpuscular volume (MCV).
Microcytic anemias are those that are distinguished by the production of red cells that are smaller than normal.
These cells are small in size as a result of decreased hemoglobin production.
Microcytic anemia is caused by: –
- Lack of globin product (thalassemia).
- Restricted iron delivery to the heme group of hemoglobin (anemia of inflammation).
- Lack of iron delivery to the heme group (iron deficiency anemia).
- Defects in heme group synthesis (sideroblastic anemias).
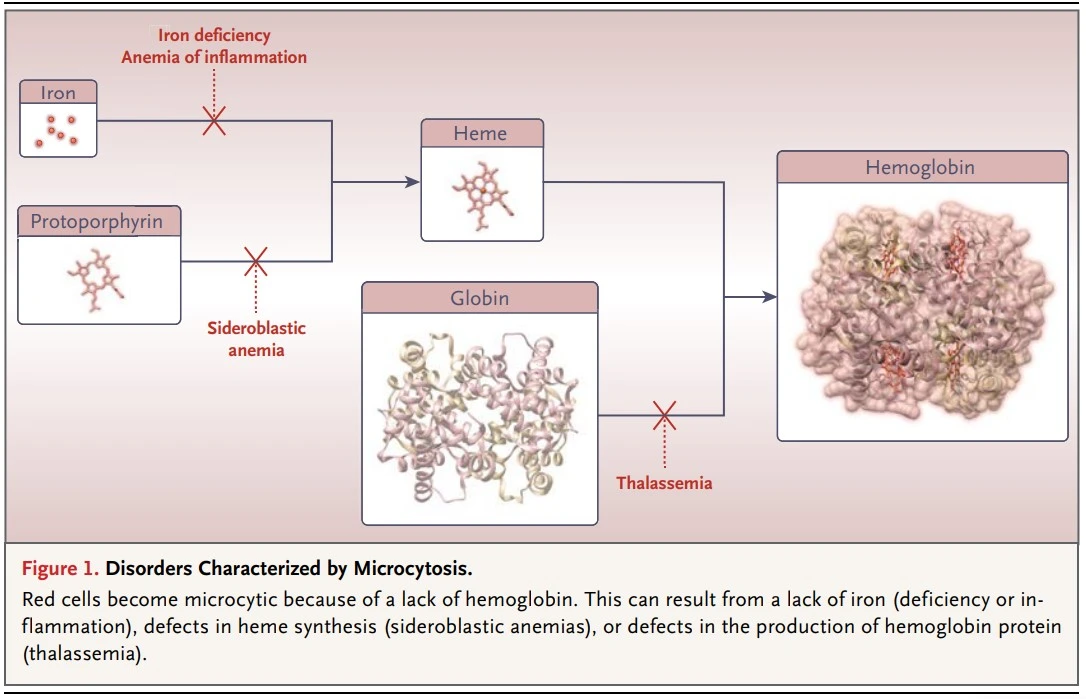
Disorders Characterized by Microcytosis
Types of Microcytic Anemia
1. Thalassemia
Thalassemia syndrome is a heterogeneous group of hereditary anemia characterized by defective synthesis of one or more globin chain subunits of Hb tetramer.
The clinical syndrome associated with thalassemia is the result of a combination of insufficient Hb development and imbalanced accumulation of globin subunits.
The factor that most influences the clinical picture is the unnecessarily high precipitation of single globin chains as a result of the lack of other partners to form the Hb molecule.
The primary cause of beta-thalassemia is an inability to synthesize β-globin as a result of a mutation in the HBB gene on chromosome 11.
Patients with α-thalassemia have an excess of β-globin and γ-globin chains as a result of defects in the HBA1 and HBA2 genes on chromosome 16.
There are four distinct subtypes of α-thalassemia: trait 1, trait 2, hemoglobin H disease, and hemoglobin Bart’s.
Patients with the trait thalassemia exhibit variable microcytosis and no or very mild anemia, which are both more pronounced in those with the trait-2 form.
Hemoglobin H disease is caused by three α-chain gene deletions or mutations and is characterized by more pronounced anemia, often with a hemolytic component.
Lack of production of the α-chain in hemoglobin Bart’s causes hydrops fetalis due to the lack of fetal and adult hemoglobin production.
β-Thalassemia is widespread in Southeast Asia and the Mediterranean region. Patients can either be homozygous (thalassemia major) or heterozygous (thalassemia minor) for the defective hemoglobin chain because there is only one copy of the hemoglobin chain on chromosome 11.
Some patients are homozygous for β-chain mutations but still have residual β-chain synthesis, resulting in an intermediate phenotype (thalassemia intermedia).
Thalassemia minor patients have mild microcytic anemia.
Thalassemia major manifests as severe transfusion-dependent anemia shortly after birth.
As the name suggests, thalassemia intermedia patients can present with anemia that is slightly more severe than that in thalassemia minor patients or anemia that is transfusion dependent.
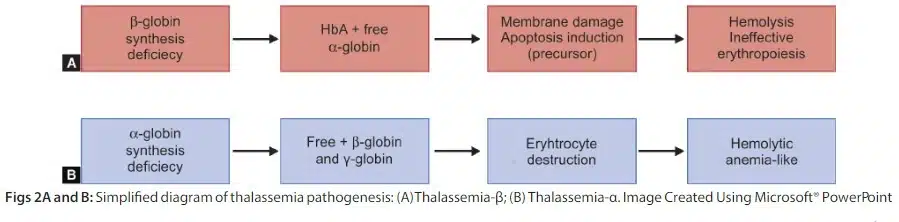
Thalassemia Pathogenesis
2. Anemia of Inflammation
Microcytic anemia is frequently associated with inflammatory states.
This anemia has two distinct causes: –
- Inflammatory cytokines suppress erythropoietin production in the kidneys, which lowers red blood cell production.
- Microcytosis can be caused by a lack of iron for developing red cells.
The lack of iron is largely due to the protein hepcidin, an acute-phase reactant that leads to both reduced iron absorption and reduced release of iron from body stores. (Read more about Anemia of Inflammation)
3. Iron Deficiency Anemia
Iron deficiency anemia is the most prevalent type of anemia.
It is not surprising that a lack of iron has effects other than anemia because iron is present in many essential proteins in cells, including cytochromes and myoglobin, in addition to playing a critical role as an oxygen carrier in the heme group of hemoglobin.
Women are more likely than men to suffer from an iron deficiency due to the obligate loss of iron during menstruation. All women lose between 1 and 3 mg of iron daily on average, and dietary intake is frequently insufficient to keep a healthy iron balance. (Read more about Anemia and Menstruation).
Diagnosis of Microcytic Anemia
Microcytic cells on the blood smear can be identified because they are smaller than a lymphocyte nucleus.
Additionally, hypochromia, an increase in the size of the red cell’s central pallor, can be observed.
Microcytic cells predominate in anemias caused by inflammation and iron deficiency, whereas target cells are also noticeable in hemoglobin E disease and β-thalassemia.
Patients with β-thalassemia minor have hemoglobin levels between 10 and 13 g/dL and mean corpuscular volume between 65 and 75 fl.
The α-thalassemia trait exhibits no electrophoretic mobility. Exclusion can be used to make the diagnosis in a patient who has microcytosis but only mild or no anemia and is iron-replete.
DNA analysis is performed for an accurate diagnosis.
Hemoglobin H disease can be identified by the presence of hemoglobin H (a tetramer of β chains) on electrophoresis along with severe microcytosis.
Patients with hemoglobin H disease may also exhibit hemolysis and have splenomegaly when physically examined.
Anemia of inflammation is currently diagnosed through exclusion.
The diagnosis is supported by three findings: –
- Erythropoietin levels in anemic patients that have not been increased appropriately
- preserved renal function and sufficient iron reserves.
- No other underlying cause of the anemia.
The serum ferritin assay is currently the most effective and economical test for the diagnosis of iron deficiency, given the limitations of other tests.
With severe iron deficiency, the mean corpuscular volume is low, but coexisting diseases like liver disease may blunt the decrease in red-cell size.
Patients with anemia of inflammation have low serum iron levels, which can be elevated by oral iron intake.
An increased total iron-binding capacity is specific for iron deficiency, but because it is decreased by aging, inflammation, and poor nutrition, it has a low sensitivity.
Treatments of Microcytic Anemia
1. Thalassemia Treatment
Chronic transfusions will help maintain growth and development in children born with severe forms of thalassemia.
However, without aggressive iron chelation, endocrine failure will occur, and most people will die from iron overload in their second or third decade of life.
These complications can be avoided or delayed with aggressive iron chelation.
The best option for treatment, if it is available, is stem-cell transplantation because, in young patients, it will have fewer complications than other options and, if it is successful, it will avoid the need for transfusion and chelation therapy for the rest of their lives.
Iron chelation is crucial for patients who depend on transfusions.
2. Anemia of Inflammation Treatment
Eliminating the underlying cause is the most effective treatment for anemia of inflammation, but in many patients, this is not possible.
Erythropoiesis stimulating agents have been successfully used to raise the red-cell count in patients with anemia of inflammation, but their use is restricted due to their high cost and safety risks.
3. Iron Deficiency Treatment
Oral Iron Therapy → For the treatment of iron deficiency, ferrous sulfate (325 mg [65 mg of elemental iron] orally three times a day]) has traditionally been recommended.
According to several studies, elemental iron doses of 15 to 20 mg per day can be just as effective as higher doses while causing fewer side effects.
There are various oral iron preparations, but none seem to be better than the others.
Starting with a daily 325-mg dose of ferrous sulfate, which is typically the least expensive form, taken with a meal that contains meat.
Taking vitamin C (500 units with the iron pill once daily) and avoiding tea and coffee will also aid absorption.
If the side effects of ferrous sulfate are intolerable, ferrous gluconate at a dose of 325 mg per day (equivalent to 35 mg of elemental iron) can be tried.
Parenterally Administered Iron à For patients who don’t respond well enough to oral iron therapy.
Parenteral iron can increase iron stores without worrying about gastrointestinal side effects or absorption.
In any case of iron deficiency that is refractory to oral iron, parenteral iron should be considered.
Low-molecular-weight iron dextran may be the most practical and least expensive choice.
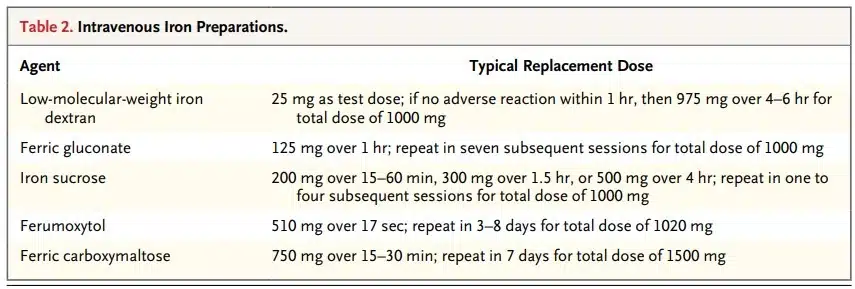
Intravenous Iron Preparations
Summary
Microcytic anemia is caused by insufficient hemoglobin (Hb) formation in erythroid precursors, resulting in a decrease in red blood cell mean corpuscular volume (MCV).
It is distinguished by the production of red cells that are smaller than normal.
These cells are small in size as a result of decreased hemoglobin production.
Microcytic anemia is caused by: –
- Lack of globin product (thalassemia).
- Restricted iron delivery to the heme group of hemoglobin (anemia of inflammation).
- Lack of iron delivery to the heme group (iron deficiency anemia).
- Defects in heme group synthesis (sideroblastic anemias).
Thalassemia syndrome is a heterogeneous group of hereditary anemia characterized by defective synthesis of one or more globin chain subunits of Hb tetramer.
Microcytic anemia is frequently associated with inflammatory states.
This anemia has two distinct causes: –
- Inflammatory cytokines suppress erythropoietin production in the kidneys, which lowers red blood cell production.
- Microcytosis can be caused by a lack of iron for developing red cells.
Microcytic cells on the blood smear can be identified because they are smaller than a lymphocyte nucleus.
Patients with β-thalassemia minor have hemoglobin levels between 10 and 13 g/dL and mean corpuscular volume between 65 and 75 fl.
The α-thalassemia trait exhibits no electrophoretic mobility. Exclusion can be used to make the diagnosis in a patient who has microcytosis but only mild or no anemia and is iron-replete.
DNA analysis is performed for an accurate diagnosis.
Hemoglobin H disease can be identified by the presence of hemoglobin H (a tetramer of β chains) on electrophoresis along with severe microcytosis.
Anemia of inflammation is currently diagnosed through exclusion.
Chronic transfusions will help maintain growth and development in children born with severe forms of thalassemia.
Eliminating the underlying cause is the most effective treatment for anemia of inflammation, but in many patients, this is not possible.
Oral Iron Therapy for the treatment of iron deficiency, ferrous sulfate (325 mg [65 mg of elemental iron] orally three times a day]) is recommended.
How useful was this post?
Click on a star to rate it!
Average rating 0 / 5. Vote count: 0
No votes so far! Be the first to rate this post.
I'm sorry that this post was not useful for you!
Let me improve this post!
Tell me how I can improve this post?
References
- (PDF) microcytic anemia: A brief overview – researchgate. Retrieved July 8, 2022, from ResearchGate
- TG;, D. L. Microcytic anemia. The New England journal of medicine. Retrieved July 8, 2022, from PubMed
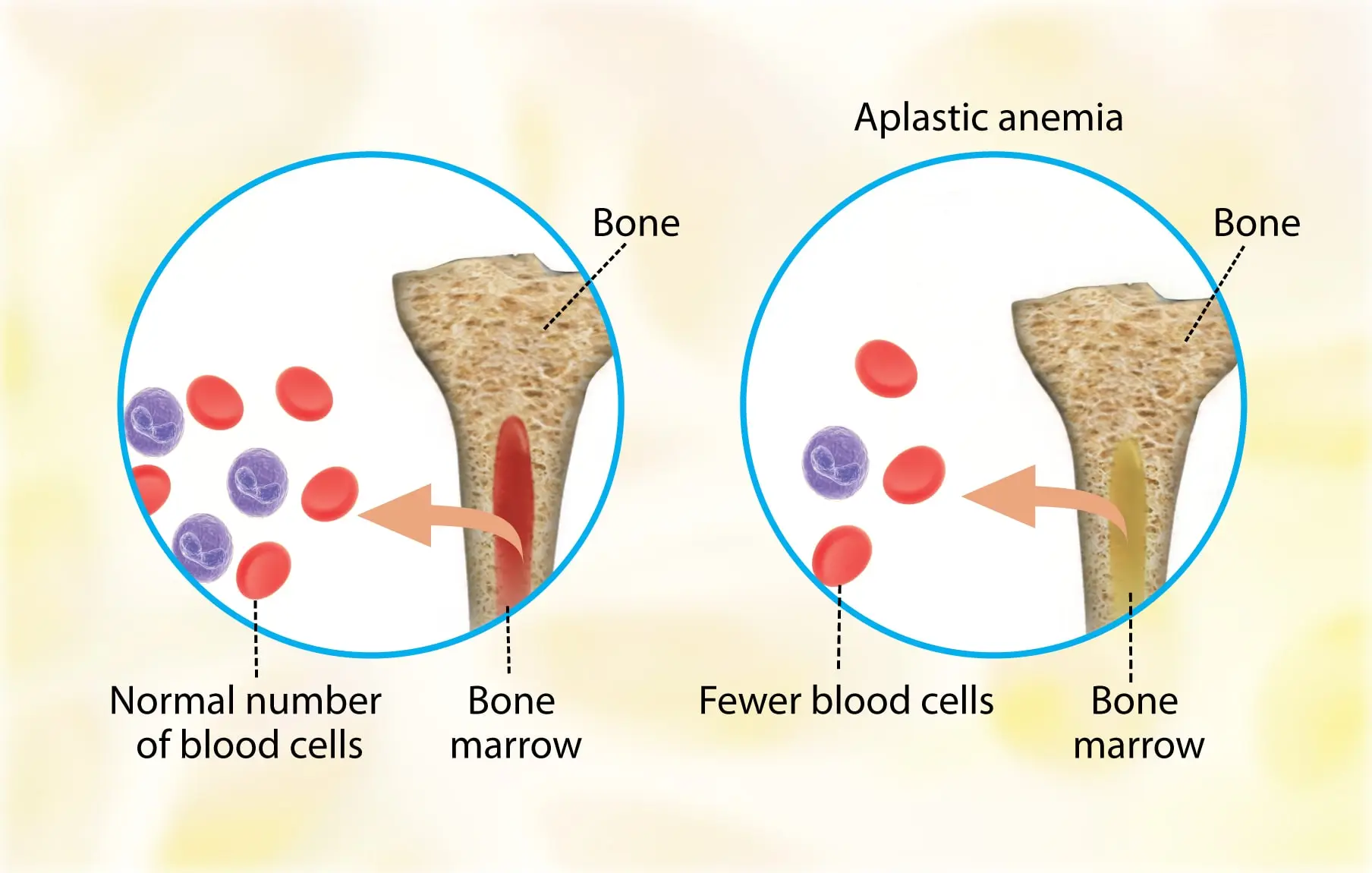
Aplastic Anemia | Causes, Symptoms, Diagnosis and Treatments
Aplastic anemia (AA) is the syndrome of chronic primary hematopoietic failure caused by injury, which results in...
Read MoreIron is involved in many neurological activities, and its deficiency has been linked to anxiety and depressive symptoms,...
Read MoreSideroblastic anemia refers to a group of inherited and acquired anemias of ineffective erythropoiesis that are characterized...
Read MoreRecent Posts
-
SAPHO Syndrome | Causes, Symptoms, Diagnosis & Treatments
-
Systemic Lupus Erythematosus | Causes, Symptoms & Treatments
-
Gastric Ulcers | Causes, Symptoms, Complications & Treatments
-
Wiskott-Aldrich Syndrome | Causes, Symptoms & Treatments
-
The 4 Stages of HIV Infection | Ultimate Guide
-
Good’s Syndrome | Symptoms, Causes & Treatments
-
Acquired Angioedema | Causes, Symptoms & Treatments
-
Rheumatoid Arthritis | Symptoms, Causes, Diagnosis & Treatments
-
Acute Pancreatitis | Causes, Symptoms, Diagnosis and Treatments
-
Pernicious Anemia | Causes, Symptoms, Diagnosis and Treatments
-
Sickle Cell Anemia | Causes, Symptoms, Diagnosis and Treatments
-
Aplastic Anemia | Causes, Symptoms, Diagnosis and Treatments
-
Diamond Blackfan Anemia | Causes, Diagnosis and Treatments
-
Sideroblastic Anemia | Causes, Symptoms & Treatments
-
Organic Dust Toxic Syndrome (ODTS) | Symptoms, and Treatments