Microcytic anemia is caused by insufficient hemoglobin (Hb) formation in erythroid precursors, resulting in a decrease in...
Read MoreHemolytic Anemia | Causes, Symptoms and Treatments
What is Hemolytic Anemia?
Hemolytic anemia is a type of anemia that is caused by the breakdown of red blood cells, an increase in hemoglobin catabolism, a decrease in hemoglobin levels, and an increase in efforts of bone marrow to regenerate products.
Based on mean corpuscular volume (MCV), anemia is often divided into microcytic, normocytic, and macrocytic subtypes.
Hemolytic anemia is classified as normocytic anemia if the MCV is between 80 and 100 fL.
Causes of Hemolytic Anemia
Hemolytic anemia has many causes, which can be categorized into: –
- Acute and chronic disease.
- Immune vs. non-immune mediated.
- Intravascular or extravascular.
- Inherited or acquired.
- Intracorpuscular or extracorpuscular.
Intracorpuscular causes are those that occur within the red blood cell itself.
When the solubility of hemoglobin changes, a red blood cell can be internally damaged (hemoglobinopathy).
Sickle cell disease (SCD) and thalassemia are two examples of hemoglobinopathies.
The most frequent cause of hereditary hemolytic anemia is thalassemia, which is brought on by a partial or complete lack of synthesis of one of the major alpha or beta globin chains of hemoglobin A.
Membranopathies include hereditary spherocytosis (HS) and hereditary elliptocytosis (HE).
Several RBC enzymopathies cause non-spherocytic hemolytic anemia by altering the shape of RBCs.
Extracorpuscular causes are defects caused by external factors such as mechanical, immune-mediated, or infectious factors.
RBC transfusions have the potential to result in acute and delayed hemolytic reactions.
Microthrombi, fibrin, or valve shearing forces can all cause mechanical trauma to RBCs.
RBCs are known to be destroyed by pathogens like malaria and babesiosis, as well as by drugs like dapsone, which can be used to treat these illnesses but also has oxidant potential.
Pathophysiology
In hemolytic anemia, RBCs are destroyed. Red blood cells typically live for 120 days.
This process can be chronic and occur over time, or it can be acute and life-threatening.
It is further subdivided according to whether the hemolysis occurs intravascularly or extravascularly.
Red blood cells will become sequestered and undergo phagocytosis if they are unable to change shape as they move through the spleen.
Hemoglobinopathies like sickle cell disease exhibit this.
Destruction can also occur as a result of inherited protein deficits.
Signs and Symptoms of Hemolytic Anemia
A patient with anemia may present with the followings: –
- Shortness of breath.
- Weakness.
- Fatigue.
- Arrhythmias such as tachycardia, or can present asymptomatic.
- Jaundice or hematuria are additional signs of anemia brought on by cell destruction or hemolysis.
Long-lasting symptoms may even be accompanied by lymphadenopathy, hepatosplenomegaly, or cholestasis.
Consider hemolytic uremic syndrome if a patient has diarrhea and hemolytic anemia.
Hematuria and lab results that are indicative of hemolytic anemia may be signs of paroxysmal nocturnal hemoglobinuria (PNH).
Complications
Multiple organ systems in the body can be affected by hemolytic anemia.
When RBCs are destroyed, their byproducts set off a chain reaction that leads to additional complications.
Chronic hemolysis in SCD reduces the amount of oxygen that can be delivered, causing tissue hypoxia.
Since more complications are being studied as a result of the toxic effects of circulating free hemoglobin and iron, the risk of ischemia and thrombotic complications can be seen in any case of hemolysis.
In people with paroxysmal nocturnal hemoglobinuria (PNH), thromboembolism is the leading cause of death. 15% to 44% of these patients will experience at least one thromboembolic event.
Both thalassemia and SCD have a hypercoagulable state that is caused by an abnormal phospholipid membrane asymmetry that has been associated with accelerated hemolysis and thrombosis.
Excess hemoglobin and iron from hemolysis can also cause kidney complications.
Many people with HS are not diagnosed until they are adults when they start to exhibit complications. They frequently exhibit recurrent cholelithiasis, and the most severe cases are frequently found to be recessive—require frequent blood transfusions.
Diagnosis
It is simple to make the diagnosis of hemolysis, which is based on the presence of anemia and sustained reticulocytosis without bleeding.
Additional findings may include marrow erythroid hyperplasia, elevated LDH, free hemoglobin, and unconjugated bilirubin, as well as decreased haptoglobin, hemopexin, hemosiderinuria, and decreased SICr red cell half-life.
Hemoglobinemia, hemoglobinuria, and hemosiderinuria occur only in the presence of severe and rapid intravascular hemolysis.
In almost all cases of hemolytic anemia, the red cell morphology is abnormal. However, in some circumstances, the morphologic abnormality may be diagnostic of the underlying condition.
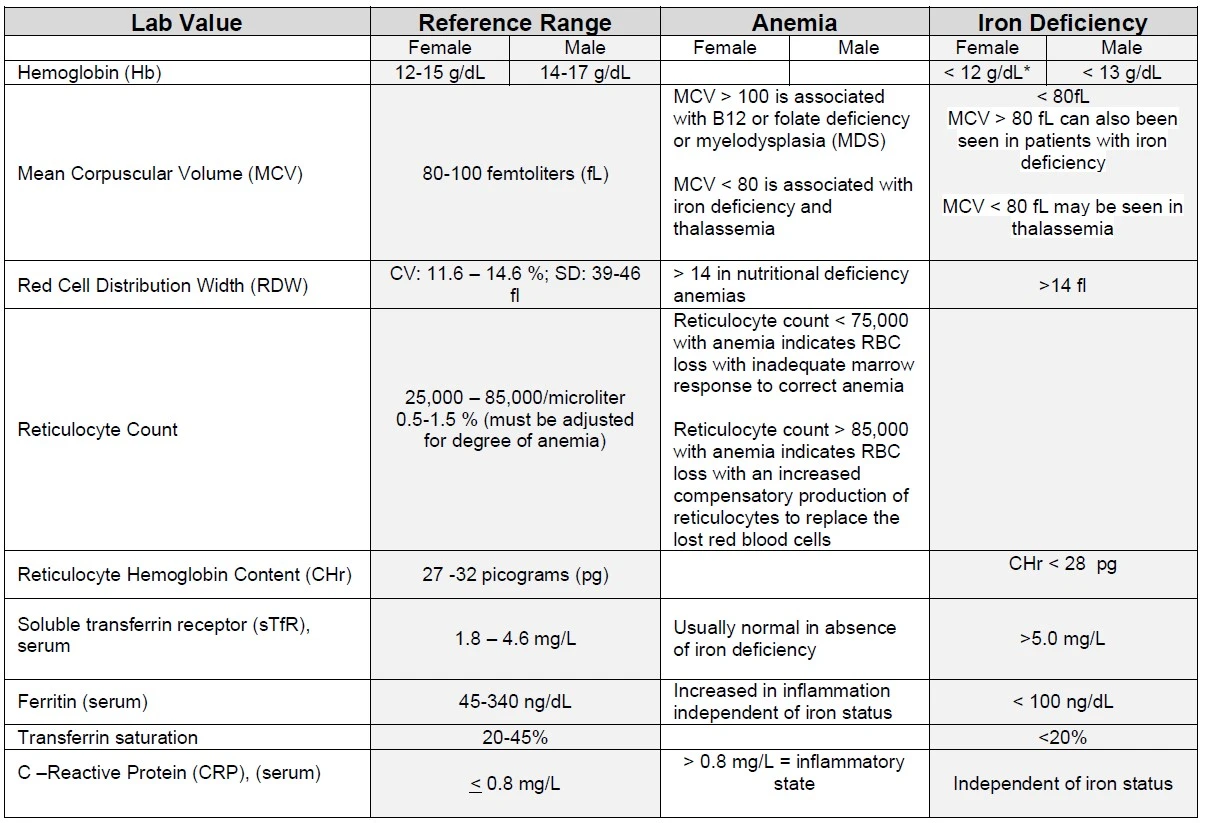
Lab Values
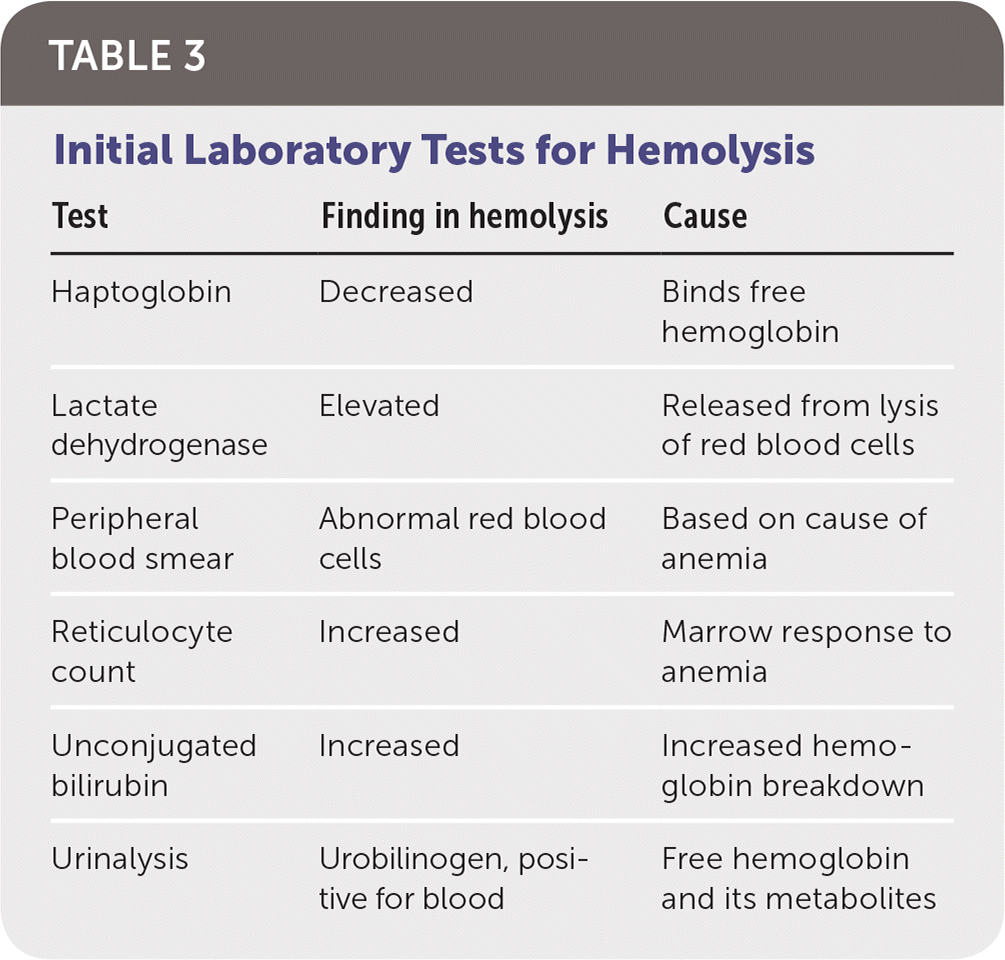
Initial Laboratory Tests for Hemolysis
Treatment of Hemolytic Anemia
Depending on the severity of the illness, immediate interventions such as blood transfusions, plasmapheresis, or diuresis may be required.
When there is severe anemia, especially when there is active bleeding, blood transfusions are always the mainstay of treatment.
More specific treatment modalities may be used if hemolysis is the known cause of anemia or if no urgent intervention is required. However, depending on the cause, the treatment will always differ.
Direct antiglobulin (Coombs) testing can be used to distinguish between immune and non-immune causes of hemolysis if the initial cause is unclear.
Blood transfusions, hydroxyurea, erythropoiesis-stimulating agents, and bone marrow transplants are potential, effective options for patients with SCD.
When G6PD deficiency is ruled out, a blood smear should be performed because it can be performed more quickly than an assay.
Furthermore, there is a chance that an assay will result in a false negative while the smear still suggestive of G6PD deficiency.
Patients must avoid medications and foods that will worsen the oxidative process once the diagnosis has been made.
Splenectomy, steroids, monoclonal antibodies, or immunosuppressants have been used as late-stage treatment options for autoimmune hemolytic anemias, HS, and SCD.
Summary
Hemolytic anemia is a type of anemia that is caused by the breakdown of red blood cells, an increase in hemoglobin catabolism, a decrease in hemoglobin levels, and an increase in efforts of bone marrow to regenerate products.
It has many causes that include acute and chronic disease, immune vs. non-immune mediated, intravascular or extravascular, inherited or acquired and, intracorpuscular or extracorpuscular.
A patient with anemia may present shortness of breath, weakness, fatigue and arrhythmias such as tachycardia, or can present asymptomatic.
Jaundice or hematuria are additional signs of anemia brought on by cell destruction or hemolysis.
Long-lasting symptoms may even be accompanied by lymphadenopathy, hepatosplenomegaly, or cholestasis.
Multiple organ systems in the body can be affected by hemolytic anemia.
When RBCs are destroyed, their byproducts set off a chain reaction that leads to additional complications.
It is simple to make the diagnosis of hemolysis, which is based on the presence of anemia and sustained reticulocytosis without bleeding.
Additional findings may include marrow erythroid hyperplasia, elevated LDH, free hemoglobin, and unconjugated bilirubin, as well as decreased haptoglobin, hemopexin, hemosiderinuria, and decreased SICr red cell half-life.
Depending on the severity of the illness, immediate interventions such as blood transfusions, plasmapheresis, or diuresis may be required.
When there is severe anemia, especially when there is active bleeding, blood transfusions are always the mainstay of treatment.
More specific treatment modalities may be used if hemolysis is the known cause of anemia or if no urgent intervention is required. However, depending on the cause, the treatment will always differ.
How useful was this post?
Click on a star to rate it!
Average rating 0 / 5. Vote count: 0
No votes so far! Be the first to rate this post.
I'm sorry that this post was not useful for you!
Let me improve this post!
Tell me how I can improve this post?
References
- Hemolytic Anemia. Retrieved December 3, 2022, from PubMed
IA;, T. Hemolytic anemias. diagnosis and management. The Medical clinics of North America. Retrieved December 3, 2022, from PubMed
AC;, P. J. H. (n.d.). Hemolytic anemia: Evaluation and differential diagnosis. American family physician. Retrieved December 3, 2022, from PubMed
Iron deficiency anemia (IDA) is the most common cause of anemia and the most treatable of all...
Read MoreSickle Cell Anemia | Causes, Symptoms, Diagnosis and Treatments
Sickle cell anemia is an inherited condition of the globin chains that leads to hemolysis and chronic organ damage.
Read MoreDiamond Blackfan anemia (DBA) is a form of congenital anemia that is characterized by pure red cell...
Read MoreRecent Posts
-
SAPHO Syndrome | Causes, Symptoms, Diagnosis & Treatments
-
Systemic Lupus Erythematosus | Causes, Symptoms & Treatments
-
Gastric Ulcers | Causes, Symptoms, Complications & Treatments
-
Wiskott-Aldrich Syndrome | Causes, Symptoms & Treatments
-
The 4 Stages of HIV Infection | Ultimate Guide
-
Good’s Syndrome | Symptoms, Causes & Treatments
-
Acquired Angioedema | Causes, Symptoms & Treatments
-
Rheumatoid Arthritis | Symptoms, Causes, Diagnosis & Treatments
-
Acute Pancreatitis | Causes, Symptoms, Diagnosis and Treatments
-
Pernicious Anemia | Causes, Symptoms, Diagnosis and Treatments
-
Sickle Cell Anemia | Causes, Symptoms, Diagnosis and Treatments
-
Aplastic Anemia | Causes, Symptoms, Diagnosis and Treatments
-
Diamond Blackfan Anemia | Causes, Diagnosis and Treatments
-
Sideroblastic Anemia | Causes, Symptoms & Treatments
-
Organic Dust Toxic Syndrome (ODTS) | Symptoms, and Treatments